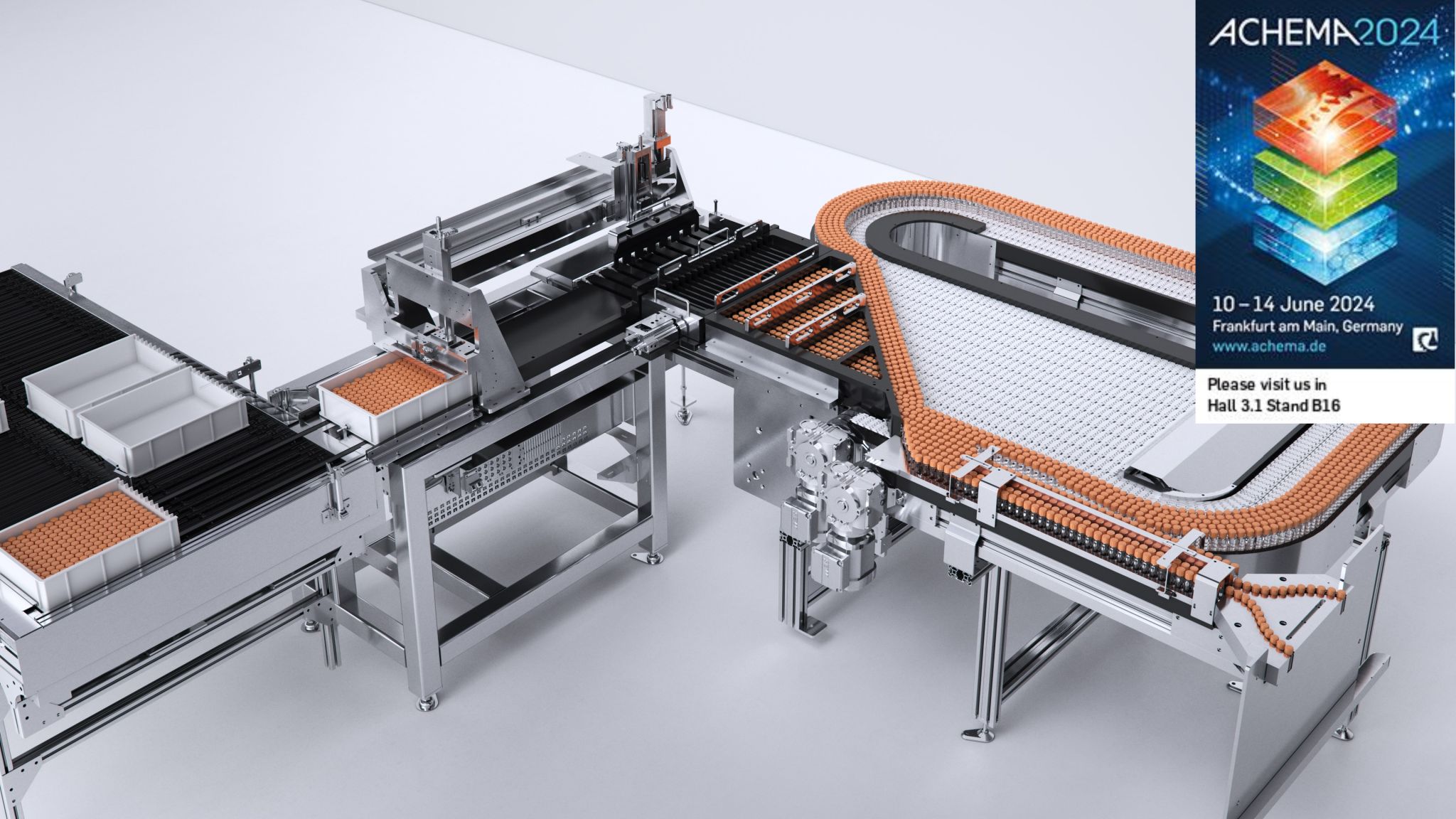
ADS, or Automated Distribution Systems, are designed for the pharmaceutical industry to handle bulk drug substances such as products, media, buffers, and more, transferring them into single-use containers through bagging and/or bottle filling processes.
Discover your next bulk distribution solution with our state-of-the-art Automated Distribution Systems (ADS). Our cutting-edge technology ensures a seamless and precise distribution process for a variety of liquid substances, including final products, media, buffer, and more.
Key Features
- Consistency: Achieve unparalleled consistency through the use of validated and configurable recipes. Our ADS ensures no variation in the distribution process steps from batch to batch, providing reliability and adherence to quality standards.
- Repeatability: Benefit from automated regulation of distributed volumes, enhancing the reproducibility of your processes with uniform and coherent results.
- Accuracy: Precision is central. Our ADS is engineered to deliver exceptional accuracy in every distribution task. From small to large volumes, trust in the accuracy of our system for reliable and error-free results.
- Data management: Compliance with regulations and guidance regarding data is paramount to the industry. Our ADS is prepared for batch records and audit trails management, ensuring your processes align with regulatory requirements.
Choose ADS for a distribution solution that meets the stringent demands of the pharmaceutical industry at both process development and production scale stages. Our commitment to consistency, repeatability, and accuracy ensures your processes are optimized for efficiency and compliance.
Explore the possibilities with ADS. Contact us today to enhance your pharmaceutical bulk drug substance distribution.